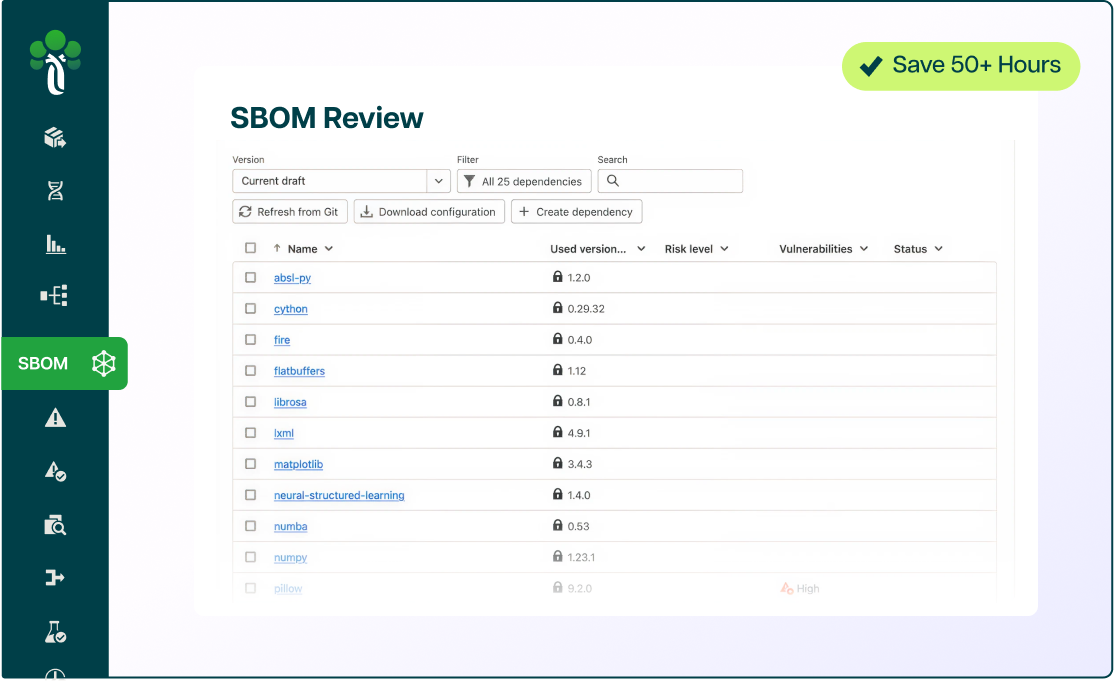
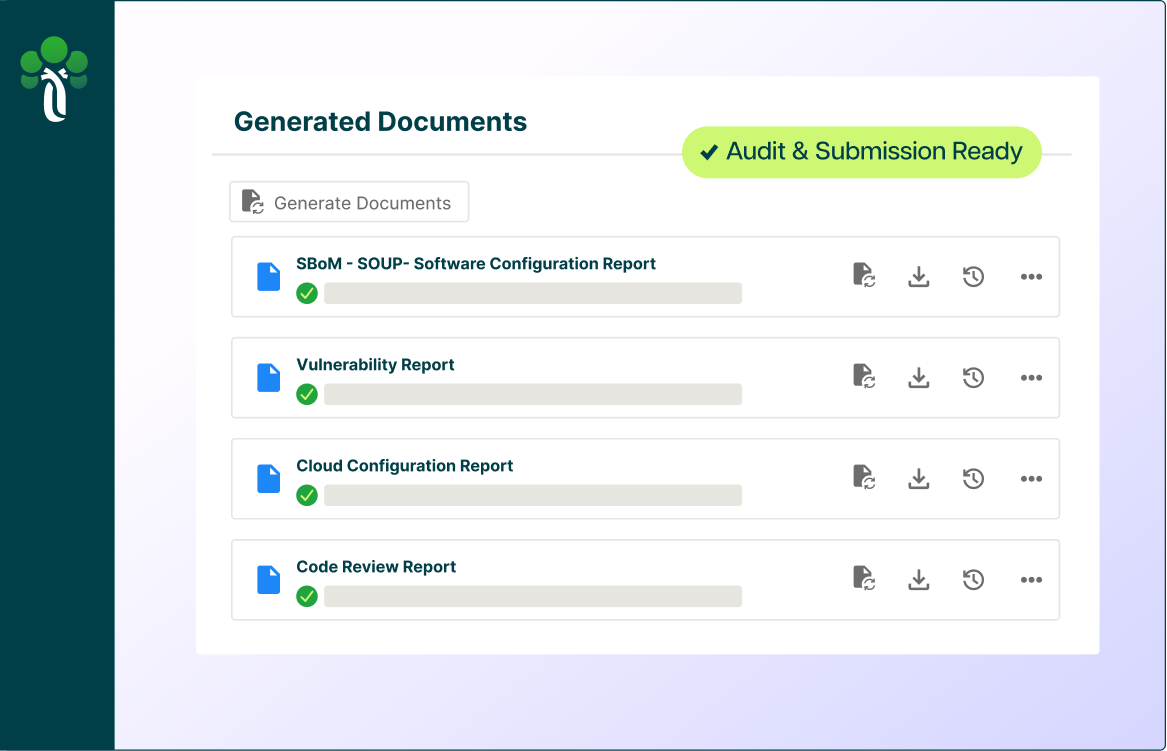
Accelerate the way you build innovative products in the AI era.


















AI and Your Product Maturity Model
Enterprise-Grade Expertise
Faster Time to Value
Built for Scale
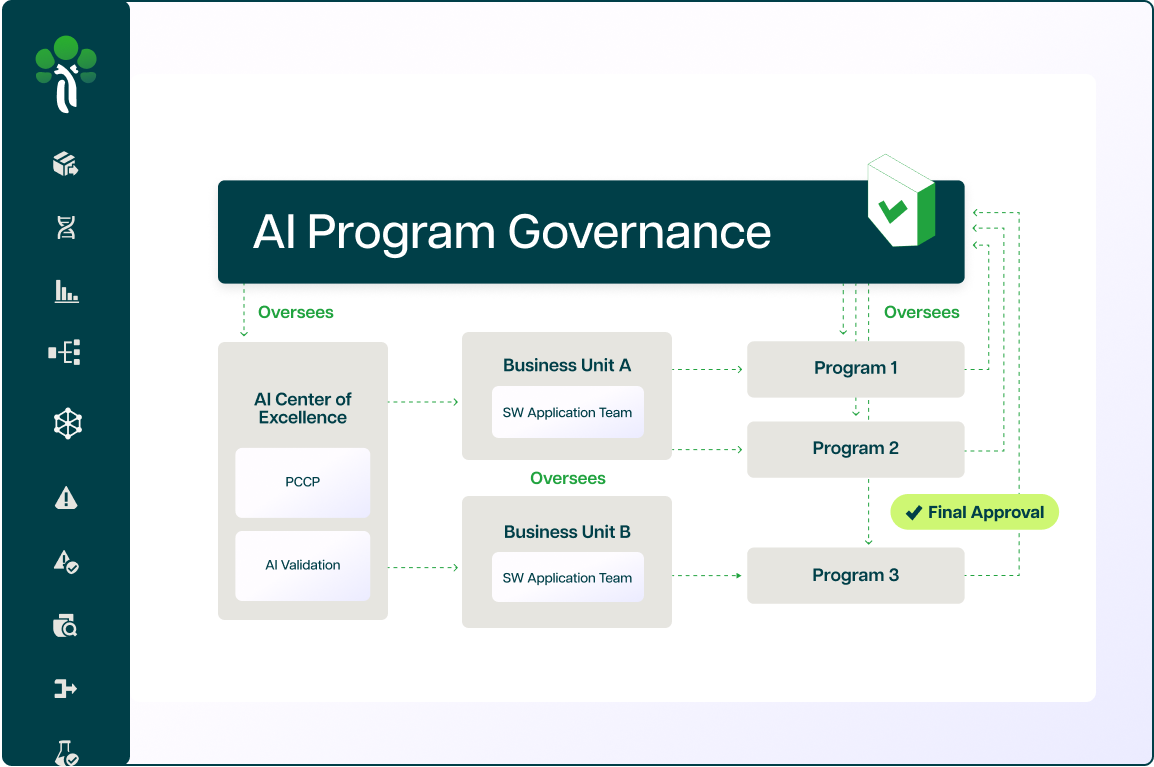
Our framework for how companies build AI today
Our People Make the Difference

Erez Kaminski



Erez Kaminski
Erez Kaminski
Erez is passionate about improving patient care and health outcomes with software solutions. Over the last decade, Erez worked in industries including computational mathematics, biotech, and energy, helping build monitoring systems for pharmaceutical equipment and AI for medication management. Before Ketryx, Erez worked with Amgen, the world’s largest biotechnology company, as the head of AI/ML for their medical device division and with Wolfram Research, the builders of Mathematica and Wolfram|Alpha. Erez holds a Master of Science in Electrical Engineering and Computer Science and a Master of Business Administration from the Massachusetts Institute of Technology.

Jenn Dixon



Jenn Dixon
Jenn Dixon
Jenn leads quality and regulatory client engagements at Ketryx, where she provides coaching and support for clients wishing to adopt the best Software Medical Device practices. Jenn brings extensive expertise in quality assurance, regulatory affairs, and project management from previous roles in the MedTech industry with a proven ability to develop and apply best practices that optimize both teams and processes.
Prior to Ketryx, Jenn was Director of QA/RA at Omniscient Neurotechnology, overseeing global regulatory submissions, maintaining ISO 13485 compliance, and managing cross-functional teams in areas such as post-market surveillance and Good Machine Learning Practices (GMLP). Jenn also served as a Design Assurance Specialist and Technical Project Manager, where she excelled in risk management, audit readiness, and leading agile development teams to deliver innovative solutions.
Jenn began her career at Synaptive Medical, supporting ISO 13485 compliance transitions and leading UDI implementation. She holds a Bachelor’s degree in Engineering from the University of Toronto.

Paul Jones



Paul Jones
Paul Jones
Paul is a world-renowned software safety expert who joined Ketryx following 25 years at the Food and Drug Administration (FDA). He helped create the FDA’s approach to safety-critical software and medical devices and founded the FDA’s software engineering lab. While holding committee positions with groups that handled medical software safety standards like ISO 13485, ISO/IEC 62304, and ISO 14971, he reviewed over 300 devices, carried out numerous inspections, and provided training to FDA staff on software quality, risk management, and software engineering. Prior to the FDA, he worked 20 years as a systems/software engineer for companies like Ford Motor, Electronic Data Systems, Honeywell, and SAIC. He holds a Master of Science degree in Computer Engineering from Loyola University, Maryland.

Lee Chickering



Lee Chickering
Lee Chickering
Lee Chickering is the Director of Quality at Ketryx and an expert in quality assurance and regulatory compliance, specializing in bridging quality management and customer success to drive operational excellence in the life sciences industry. With a diverse background spanning manufacturing, project management, and compliance at companies like Amgen, he has led the implementation of Quality Management Systems (QMS) aligned with ISO 13485, ISO 14971, and IEC 62304. Passionate about advancing quality in life sciences, he thrives on collaborating with organizations to enhance efficiency, compliance, and innovation.

Matt Dumouchel



Matt Dumouchel
Matt Dumouchel
Matt brings over a decade of experience in digital manufacturing and process excellence to help life sciences teams modernize their operations and achieve continuous compliance. At Ketryx, he supports clients in implementing connected systems that enhance traceability, automate documentation, and accelerate release cycles. Prior to Ketryx, Matt led digital transformation initiatives at Tulip Interfaces and Amgen—advancing GxP-validated electronic lab notebooks, integrating 25+ classes of lab instruments, and delivering multi-million-dollar efficiency gains through Lean and data-driven process improvements. His leadership in operational excellence and digital systems enables organizations to connect R&D, Manufacturing, and Quality for faster, safer innovation. Matt holds dual Master’s degrees from MIT as a Leaders for Global Operations Fellow.

Bailey Canter



Bailey Canter
Bailey Canter
Bailey brings a unique blend of systems engineering, agile leadership, and enterprise software delivery expertise to help life sciences organizations build safer products faster. As Director of Solutions at Ketryx, she partners with enterprise clients to implement connected compliance systems that unify R&D, Quality, and IT workflows. Before joining Ketryx, Bailey spent three years at Amwell, where she led software deployments and served as Scrum Master for multiple healthcare platform teams, driving operational excellence across DevOps, QA, and client support. Earlier in her career at Raytheon Technologies, she worked on radar systems engineering and model-based design for defense programs. Her cross-industry experience enables her to bridge technical, operational, and regulatory domains to deliver scalable, compliant solutions.

Gabriel Pascualy



Gabriel Pascualy
Gabriel Pascualy
Gabriel has deep expertise in device security and cyber-physical systems. He has a patent pending for “Systems and Methods for Identifying Fault Vulnerabilities and Inserting Fault Countermeasures” and has been a featured speaker at Med Device Boston and the INCOSE Healthcare Systems Engineering Conference. While at the MITRE Corporation, he managed the Cybersecurity Innovation portfolio and led research teams focused on device security and cyber-physical systems. He later joined Amgen, where he applied AI and Machine Learning to improve drug manufacturing operations. Gabriel has also created technology solutions for Fortune 500 MedTech companies to enhance cybersecurity postures, automate SBOM documentation, and accelerate software development lifecycles by shifting left. Gabriel holds a Master of Science in Electrical Engineering and Computer Science and a Master of Business Administration from the Massachusetts Institute of Technology. He now serves as the Head of Product at Ketryx, a platform purpose-built for life sciences teams to improve quality and release software faster.

Carlton O'Neal



Carlton O'Neal
Carlton O'Neal
Carlton combines deep operational expertise with product strategy to help life sciences organizations improve quality, compliance, and efficiency. At Ketryx, he leads initiatives that bridge customer success, product development, and implementation. Before joining Ketryx, Carlton spent over four years at Amgen, where he advanced from manufacturing to senior process development roles. His experience in both regulated operations and product delivery gives him a unique perspective on how to connect R&D and Quality teams in practice. Carlton holds a Bachelor’s degree in Biochemistry from Occidental College, where he was Varsity Football Captain.
What our MedTech clients are saying

“Ketryx's expertise and dedication to customer success have made them an indispensable partner, and we look forward to continuing our collaboration to achieve even greater operational excellence.”

DeepHealth - Radnet
The experts medtech teams trust