
How Regulated Teams Release Every Two Weeks
Table of Contents
Compliance shouldn’t mean slow.
The big question regulated teams are asking is this: How do we release faster?
Most teams in regulated industries feel stuck in release cycles that stretch for months, weighed down by manual documentation, rigid approvals, and review processes that take longer than the development work itself. But the reality is that faster cycles are not only possible—they’re already happening. Companies using Ketryx have shown they can move to biweekly release rhythms while still passing audits. And these companies find that moving faster actually helps them develop even safer products, since they are able to release a patch or hotfix faster than before.
Here’s how they do it:
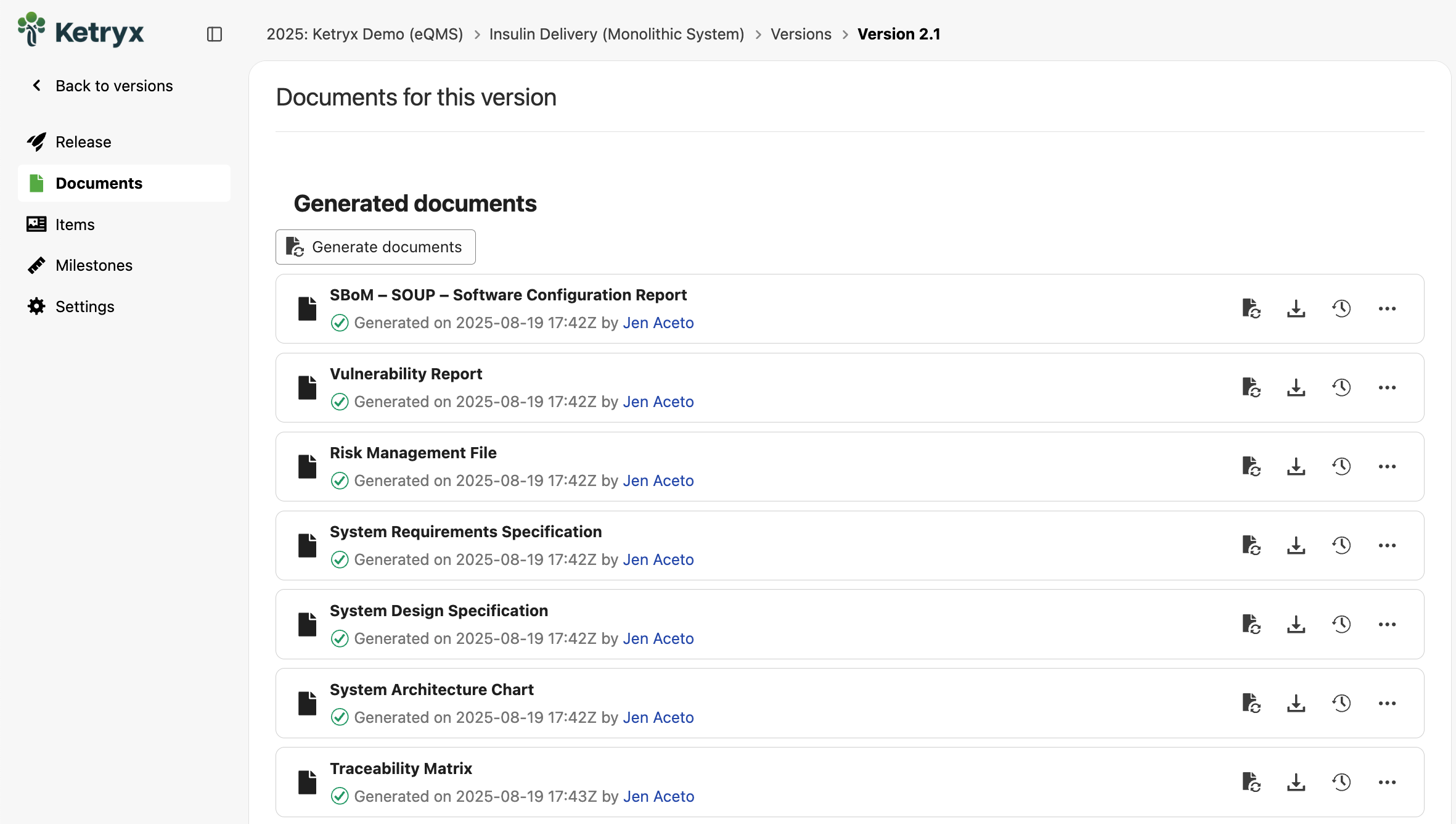
1. Automatically Generated Release Docs
Traditional release cycles often grind to a halt because teams spend days (sometimes months!) assembling Word docs, spreadsheets, and PDFs to show auditors what changed between versions. Documentation is the single biggest bottleneck.
Ketryx automatically generates your release package, including traceability matrices, requirements history, test results, and risk documentation. It’s version-controlled, audit-ready, and created in minutes instead of weeks. As teams make changes (e.g., a new requirement or updated test), Ketryx logs, traces, and validates in real time. At release, you click “Generate Package,” and the full release doc is ready.
2. Release Small, Release Often
Large scale, major releases create risk. If something breaks, you’re re-testing and re-documenting a mountain of changes. Smaller, frequent releases spread risk and reduce rework. Instead of batching changes into one major/minor release, ship incremental fixes. This makes reviews shorter, tests lighter, and releases more predictable. Ketryx supports incremental releases by letting you scope release artifacts to only changed items. The platform handles versioning automatically, so your compliance is intact even if you release weekly.
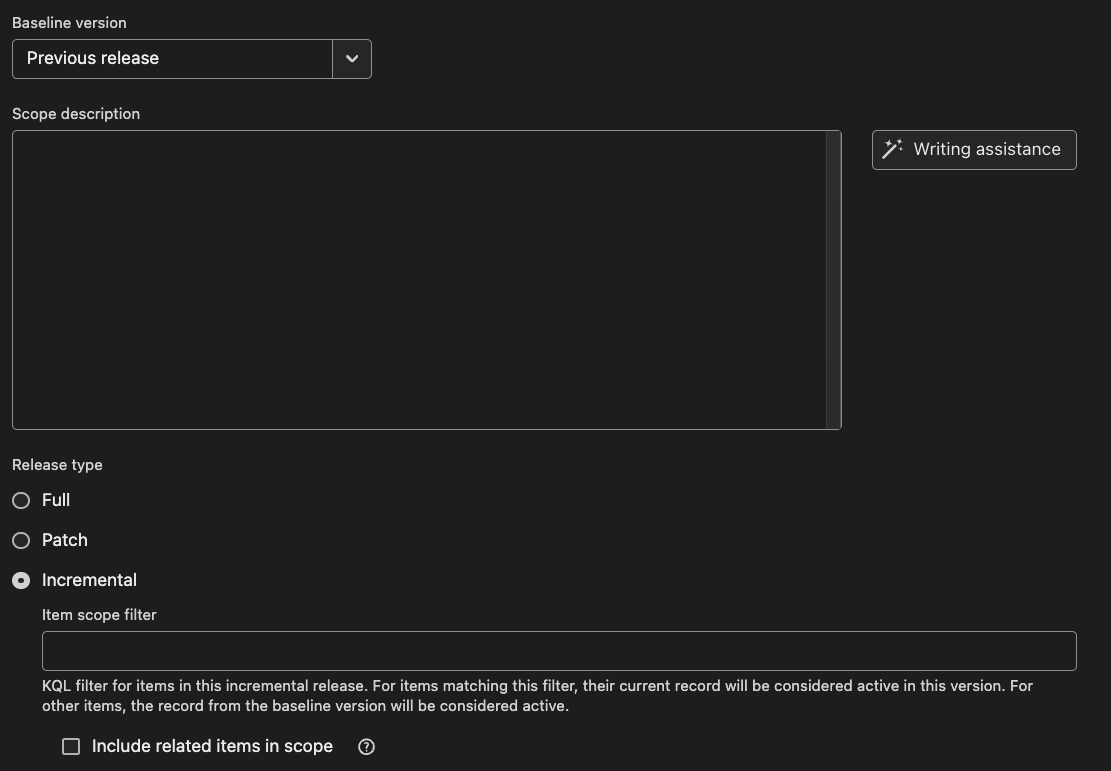
3. Break Free from Waterfall
Waterfall workflows delay testing, reviews, and risk management until the end. By then, problems are costly to fix and documentation piles up. Agile and hybrid SDLCs keep compliance in sync with development. You don’t need to wait until the end to validate. Ketryx works with your existing tools (Jira, GitHub, GitLab, Jama) and captures compliance artifacts continuously. That way, traceability builds as you go, not as a last-minute sprint.
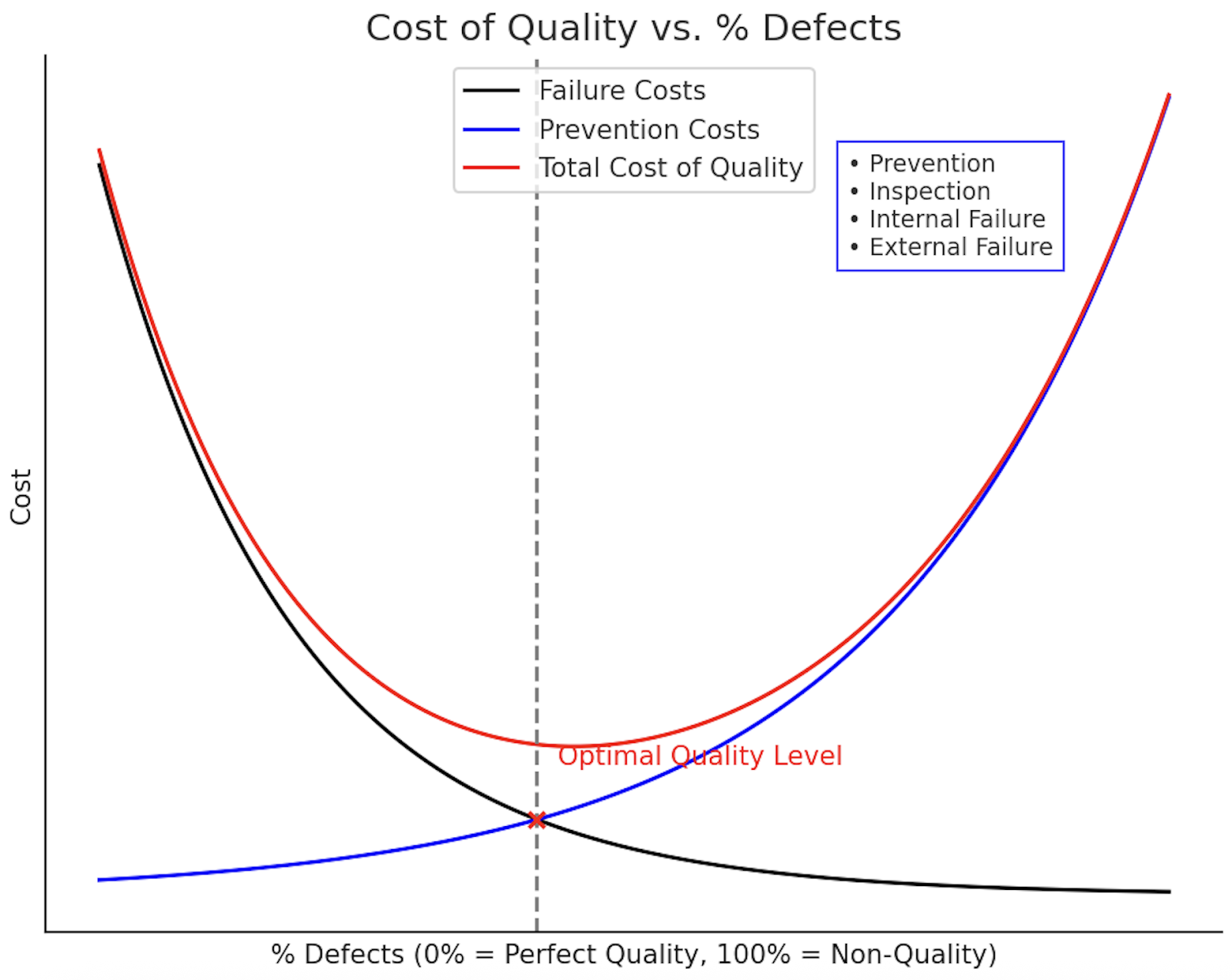
This idea ties closely to the Cost of Quality curve shown below. The longer you wait to find and fix issues, the more expensive they become. Waterfall pushes failure costs up because defects pile up until the end. By contrast, continuous compliance and prevention (what Ketryx enables) keep costs lower and quality higher.
4. AI Agents
Reviews and documentation creation eat up time. Quality professionals often spend hours on tasks AI could handle. Ketryx Agents draft requirements, flag missing traceability, review test cases, and highlight conflicts — leaving humans to focus on critical decisions. You invoke an AI Agent for item creation or review. It proposes structured content, and you accept, edit, or reject, keeping humans in control.
Think of our AI agents as compliance copilots. They don’t replace your team’s judgment, but they do handle the repetitive, low-value work that eats up your time. Agents can draft requirements and risks in the right structure, spot when a requirement doesn’t have a linked test, suggest test cases that fit your existing framework, and even flag conflicts between items.
Instead of starting from scratch or manually policing every connection, your team gets a head start. You stay in control, reviewing, accepting, or editing the agent’s suggestions, but you skip a lot of the heavy lifting.
For teams aiming for two-week release cycles, that kind of support makes a huge difference. It means less time spent pushing paperwork, and more time building and releasing software.
5. Item-Level Approvals
Document-level reviews are slow because they bundle everything together. Approvers must wade through material they’ve already signed off on. With Ketryx, every item (requirement, risk, test case) is reviewed and approved individually. By the time you compile a release doc, it’s just a formal confirmation. Approval workflows in Ketryx can be configured so reviewers sign off at the item level. Traceability ensures everything is linked and accounted for in the final package.
6. Incremental Artifact Generation
Generating a full release package every time is overkill. Most releases only touch a handful of items. Ketryx lets you generate artifacts only for changed or new items. This keeps releases lean and relevant, cutting both prep and review time. For incremental releases, you don’t want to regenerate an entire documentation package each time. That just bloats the release and adds work. Instead, teams should be creating templated release documents in Ketryx.
Here’s how it works: you create a template once that defines what a release package looks like for your organization. For example, you may want your release documentation to focus on new or changed items:

When you generate release documentation, Ketryx automatically fills that template with the relevant items.
For incremental releases, you select the scope of what’s changed or what should be carried forward. Ketryx then generates the release docs using your template, pulling in the right set of updated requirements, risks, and test results.
This approach keeps releases lean and focused, while making sure the format of your documentation is always consistent. Instead of every release being a one-off, you’re building on a stable, templated framework — which saves time, avoids formatting mistakes, and keeps auditors happy.
7. Automate Testing
Manual testing is error-prone and slow. Automated test pipelines keep development moving but often don’t sync with compliance docs. Ketryx integrates CI/CD results into your traceability matrix automatically. Every automated test result is logged against requirements and risks. Pass/fail data flows into your validation docs, no manual transfer required.
8. Continuous Risk Management
Risk handled only at major milestones leads to last-minute surprises. Regulators expect risk to be “living,” not static. Ketryx makes risk continuous. New requirements or changes should automatically trigger a risk impact assessment. When you update a requirement or test, you can configure Ketryx to associate risks and prompt mitigation or reassessment.
Ketryx Risk Management Guide shows how continuous risk reduces delays by avoiding “big bang” updates.
9. Parallel Sprints & Modern PM Methods
Teams often run multiple development streams (e.g., a bug-fix sprint alongside feature work). Without integrated compliance, this gets messy. Ketryx gives real-time traceability across parallel projects. You can branch, merge, and release without losing compliance oversight. Cross-project referencing and traceability pools (see Ketryx’s documentation on a system of systems approach) keep everything consistent, even when multiple sprints feed into a release.
10. Audit Your SDLC for Required vs. Habitual Steps
Many teams cling to “best practices” that aren’t required by regulation but drag down speed. A gap analysis is about asking a simple question: are we doing this because it is required, or because it is tradition?
Start by looking at your SDLC as it exists today and compare it to what the standards actually ask for, such as ISO 13485, IEC 62304, ISO 14971, and FDA 21 CFR 820. Some steps are non-negotiable, like maintaining traceability, running risk assessments, or validating changes. Others are just habits that have built up over time, like multi-layered sign-offs or lengthy document reviews that do not add measurable value.
Once you know which activities are mandatory and which are just slowing you down, you can streamline. Use Ketryx to align workflows to standards. Eliminate steps that don’t meaningfully impact safety or quality.
11. Rethink Your Review Culture
Reviews stall when people feel obligated to comment. That slows everything down, even when the content is acceptable. Reviews should flag real issues (noncompliance, safety risks), not personal preferences!
People feel like they need to say something in every review, even if the item in front of them already meets the standard and would pass an inspection. That instinct slows things down without improving quality.
For example, imagine a reviewer is looking at a requirement that clearly states what the feature needs to do, has linked risks, and has corresponding test coverage. It checks every box in the procedure, yet the reviewer leaves a comment suggesting a wording tweak or an alternative phrasing. Now that item goes back into circulation, a response is needed, and days get added to the timeline — all for something that doesn’t improve compliance or safety.
Ketryx helps keep reviews focused by surfacing whether an item meets the procedure, shows its traceability, and highlights gaps if they exist. If no gaps are there, the system gives reviewers the confidence to approve it as-is, instead of holding up the process for commentary that doesn’t move the needle.
12. Ketryx Can Help (Professional Services)
Even with automation, some teams want guided help to rework their SDLC. Ketryx offers professional services to help build validation plans, streamline review processes, or conduct SDLC gap analysis. Work with your account executive. Services are scoped separately unless bundled into your initial contract.
[hide-start title="Frequently asked questions"]
Q: Will auditors accept two-week release cycles?
Yes. Auditors don’t care how short your cycle is, only that compliance artifacts are accurate and traceable.
Q: Do I still need full document reviews?
Not if item-level approvals are in place. Document approvals become a formality when every item has already been reviewed.
Q: How does Ketryx handle incremental vs. full releases?
Ketryx gives you flexibility. Sometimes you need the whole picture, other times you just want to release the changes you actually made.
- Full releases are the big ones. They pull in the entire current state of your system: every requirement, risk, and test in its approved version. These are what you’d use for major updates or when you want to lock down the full baseline of your product.
- Incremental releases are for when you don’t want to drag everything along with you.
- A patch release builds on your baseline and only includes items that have been added or retired since the last release. Perfect for bug fixes or quick enhancements.
- A scoped release is even more targeted. You define the scope (say, a specific filter or feature branch) and only those items and their related records make it into the release.
- A patch release builds on your baseline and only includes items that have been added or retired since the last release. Perfect for bug fixes or quick enhancements.
Behind the scenes, Ketryx keeps everything version-controlled and enforces approvals, so whether you’re doing a full release or a quick patch, the documentation is always compliant and audit-ready. And since multiple versions can live in parallel, you can keep working on the next release while finalizing the current one without stepping on your own toes.
Q: What about SBOM and security?
Ketryx supports SBOM and component tracking, tying vulnerabilities directly to requirements and risks.
Q: Can Ketryx help re-design our SDLC?
Yes, but that typically falls under paid professional services. We’ll guide you through process optimization, but you own the final documentation.
[hide-end]

Lee Chickering is a Director of Client Operations at Ketryx and an expert in quality assurance and regulatory compliance, specializing in bridging quality management and customer success to drive operational excellence in the life sciences industry. With a diverse background spanning manufacturing, project management, and compliance at companies like Amgen, he has led the implementation of Quality Management Systems (QMS) aligned with ISO 13485, ISO 14971, and IEC 62304. Passionate about advancing quality in life sciences, he thrives on collaborating with organizations to enhance efficiency, compliance, and innovation.






